Abstract
Background: Patients with lower-risk MDS (LR-MDS) are prone to iron toxicity due to long-term iron accumulation either caused by RBC transfusions or ineffective erythropoiesis. Nontransferrin bound iron (NTBI), including labile plasma iron (LPI), are toxic iron species that may mediate cellular damage via oxidative stress. The EUMDS registry collects prospective observational data on newly diagnosed LR-MDS patients from 145 centers in 17 countries since 2008.
Methods: We analyzed serum from 247 LR-MDS patients collected at six-month intervals for ferritin, transferrin saturation (TSAT), hepcidin-25, soluble transferin receptor (sTfR) and toxic iron species (NTBI and LPI) in order to evaluate temporal changes in iron metabolism, the presence of potentially toxic forms of iron and their impact on survival, and quality of life. In addition, we measured the impact of iron chelation on the iron species levels and its impact on the outcome parameters.
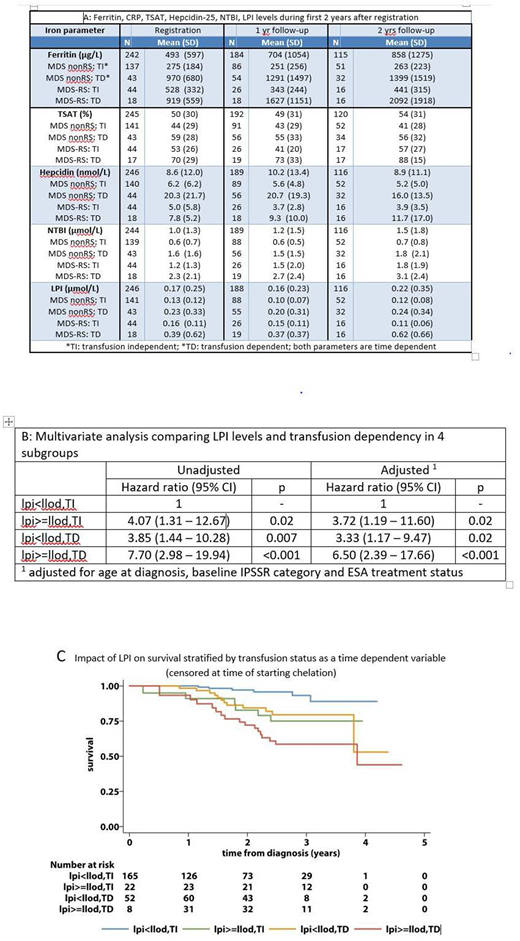
Results: The median age was 73 years (range: 37 to 95 years) and 66% were males. WHO2001 MDS-subtypes were RCMD (45%), RARS (22%), RA (18%), RAEB-1 (7%), 5q-syndrome (4%) and RCMD-RS (4%). The IPSS-R categories were: (very) low risk: 66%; intermediate risk: 11%; (very) high risk: 2% and unknown: 20%. The median EQ5D index score was 0.80 (p10 to p90: 0.52 to 1.00). The table shows iron parameters at registration, 1 and 2 years follow-up both in transfusion-dependent (TD) and transfusion-independent (TI) patients and according to: MDS-RS (RARS/RCMD-RS) or MDS Other (RA/RCMD/RAEB/5q-syndrome). Mean serum ferritin levels were increased in TD patients, compared to TI patients (Table). Increase of ferritin levels over time was high in both TD groups, but the increase was more pronounced in the RS subgroup. Elevated CRP levels (> 10mg/L) were observed in TD nonRS patients, especially during the first year after diagnosis. Markedly increased mean TSAT levels (>75%) occurred in the subgroup of TD-RS patients throughout the observation period. Hepcidin levels were most markedly elevated in TD nonRS patients at registration and remained elevated during follow-up (Table). Hepcidin levels were low in MDS-RS TI patients at all time points compared to nonRS MDS patients and decreased over time as a result of an increased (ineffective) erythropoiesis (Table). This is supported by the highest levels of STfR also noted in this patient category (data not shown). The highest NTBI and LPI levels were observed in TD-RS patients compared to the other 3 subgroups (Table). Both NTBI and LPI levels had a strong correlation (p <0.001) with TSAT. Elevated LPI levels in combination with high TSAT levels (>80%) occurred almost exclusively in patients with MDS-RS and/or previous transfusions. Both the EQ5D index score and EQ-VAS showed a negative correlation (r) with LPI levels with r = -0.09 (p = 0.028) and r = -0.07 (p=0.046) respectively. This negative effect of elevated LPI levels was most pronounced in the TD RS subgroup with a negative correlation of -0.17 for the EQ5D index score and -0.2 for the VAS score. In total 16 patients received iron chelation during the sample collection period (11 patients deferasirox, 4 patients desferioxamine and 1 patient unknown). LPI levels were normal in 14 out of the 17 samples collected during deferasirox treatment and in 2 out of 5 samples collected during desferoxamine treatment.
The Kaplan-Meier curves (Figure) demonstrate the prognostic impact of elevated LPI levels by transfusion status as a time dependent variable; once a subject had an elevated LPI level, they remained in this group. Patients were censored at time of starting iron chelation (16 patients). In a multivariate analysis comparing elevated LPI levels and transfusion dependency to the control group with undetectable LPI and no transfusion showed a significantly decreased survival in all 3 risk groups after adjustment for age at diagnosis, baseline IPSS-R category and ESA treatment status; for details, see supplementary table.
Conclusion: This study illustrates labile plasma iron species as a clinically relevant assay for identification of the toxic fraction of overt iron overload and its negative impact on HRQoL and overall survival in transfusion dependent and transfusion independent patients.
Culligan:Merck Sharp & Dohme (MSD): Honoraria; Abbvie: Other: Support to attend conferences; Takeda: Honoraria, Other: Support to attend conferences; Pfizer: Honoraria; Celgene: Other: Support to attend conferences; JAZZ: Honoraria; Daiichi-Sankyo: Other: Support to attend conferences. Garelius:novartis: Honoraria. de Witte:Celgene: Honoraria, Research Funding; Novartis: Research Funding; Amgen: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal